In the medical field, cryotherapy is used in an effort to relieve pain and swelling after soft-tissue management or surgery. Currently, researchers in the U.S. are exploring the possibilities and limitations of vital pulp cryotherapy in clinical trials. Dr. James Bahcall, who plays an important role in these investigations, is a clinical professor at the University of Illinois at Chicago. He spoke to Dental Tribune International about the studies.
Dr. Bahcall, in collaboration with other researchers, you have published an article titled “Introduction to vital pulp cryotherapy” in which the use of cold therapy in endodontics is explored. What is the history behind the use of cryotherapy in vital pulp therapy?
There has been a paradigm shift in vital pulp therapy over the last three to five years. We have gained a better understanding of pulp biology from caries involvement, and there have been new developments in bioceramic materials. We have also come to view vital pulp therapy as a permanent rather than temporary dental treatment. All of this allowed us to develop vital pulp cryotherapy. Although we did not invent cryotherapy, we were the first to bring it into endodontics for vital pulp treatment. Medicine has demonstrated since the early 1960s that cryotherapy can reduce nerve pain response, inflammation and hemorrhaging, and can help reduce a patient’s need for postoperative pain medications.
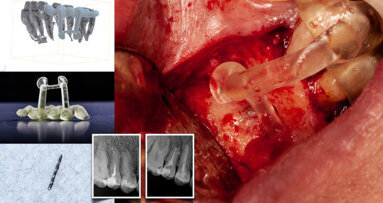
Vital pulp cryotherapy is performed when a carious lesion is removed from a tooth and there is direct or indirect exposure of the dental pulp. The cryotherapy portion of treatment involves placing sterile ice on the exposed pulp. The application of ice lowers the temperature of the tooth’s blood and nerve supply, and this has been shown clinically to reduce inflammation and post-treatment tooth pain. It is important to note that, after performing the cryotherapy procedure, 17% EDTA irrigation is applied, a bioceramic material is then placed over the directly or indirectly exposed pulp, and the tooth is restored with a permanent restorative material, such as composite or amalgam.
How is this different from classic root canal therapy?
Vital pulp cryotherapy involves treating a carious tooth while maintaining the tooth’s pulpal tissue as opposed to root canal therapy that involves removal of the entire dental pulp and replacing it with gutta-percha and sealer.
What are the benefits of vital pulp cryotherapy, and what are its limitations?
The benefits of vital pulp cryotherapy are its ability to eliminate pulpal inflammation and a patient’s tooth pain without the complete removal of the dental pulp. By maintaining the dental pulp, we are able to maintain the tooth’s strength by not having to remove root dentin, the pulp–dentin complex and the pulp’s immune defense mechanisms. Another benefit of vital pulp cryotherapy is the treatment time for the patient. Once the patient is properly anesthetized and the caries is removed, the actual time to complete the vital pulp cryotherapy portion is 10–15 minutes. In comparison, root canal therapy can take 1–2 hours. Vital pulp therapy procedures are completed in one patient treatment visit.
The limitation of vital pulp cryotherapy is that this procedure can only be performed on vital teeth that can be permanently restored with composite or amalgam immediately after the procedure. It cannot be performed with necrotic or partially necrotic pulps. A clinician cannot prepare a vital pulp cryotherapy treated tooth for a crown. The reason for this is that, once the vital pulp cryotherapy is completed, you do not want to do any further dental treatment to this tooth because you risk the possibility of restimulating the pulpal inflammation.
In your article, you conclude that further clinical studies are needed in order to establish the long-term prognosis of a pulp after vital pulp cryotherapy. What are your expectations?
As with any new dental procedure, clinical cases and studies need to be published in peer-reviewed dental literature. Vital pulp cryotherapy is no different. We have published case reports and have been conducting clinical research on vital pulp cryotherapy. Our study has found that patients have less postoperative pain immediately after treatment and maintain normal pulp vitality at six months and at one year after treatment. This is as far as our clinical study has patient recalls at this point. Our expectations are, firstly, to demonstrate that this is a valid procedure for vital pulp treatment beyond one year. Secondly, we hope to encourage our dental colleagues to publish vital pulp cryotherapy case reports and clinical research in the dental literature.
How do you think vital pulp cryotherapy will advance endodontics?
We feel that vital pulp cryotherapy will help to broaden the type of pulpal treatment that we can provide to our patients. It also will be an important treatment component in bioactive endodontic therapy. Bioactive endodontics is the future. By definition, “bioactive” means having a biological effect. Bioactive endodontics in conventional endodontic treatment includes vital pulp cryotherapy and regenerative endodontics. It involves the use of bioactive materials and the patient’s own blood to help heal, as in the case of vital pulp cryotherapy, and to replace the gutta-percha and sealer in classic root canal therapy.
Tags:
The use of digital technologies in dentistry is on the rise, a fact that Dr Andreas Kurbad is well aware of. Kurbad, who has been running his own dental ...
There are enormous differences in opinion regarding the best methods for shaping root canals. A review of the literature reveals virtually no agreement on a...
This year, Planmeca is celebrating 50 years of contributions to modern dental practice. Many of our readers will know Heikki Kyöstilä, chairman and CEO ...
Dynamic real-time surgical navigation digital imaging, diagnostics and impressions, and the use of computer-aided design/computer-aided manufacture ...
As the global conversation around oral health continues to evolve, the European Federation of Periodontology (EFP) is playing an increasingly influential ...
A main goal of endodontic treatment is to effectively irrigate the canal and thus prevent reinfection of the peri-apical tissue. Recently, a range of new ...
At a time when venerable institutions in numerous countries, such as the UK’s National Health Service, are already setting up their own digital ecosystems...
Specialised in periodontology and oral implantology, Dr Rajiv Saini is an avid lecturer in both fields. He is Editor-in-Chief of the International Journal ...
As one of the world’s largest dental equipment manufacturers, Finnish company Planmeca is committed to providing products that can be enjoyed by dental ...
BASEL, Switzerland: It is clear from the growing number of clinicians already using digital technologies that offering simple, safe and more pleasant ...
Live webinar
Tue. 24 February 2026
1:00 pm EST (New York)
Prof. Dr. Markus B. Hürzeler
Live webinar
Tue. 24 February 2026
3:00 pm EST (New York)
Prof. Dr. Marcel A. Wainwright DDS, PhD
Live webinar
Wed. 25 February 2026
11:00 am EST (New York)
Prof. Dr. Daniel Edelhoff
Live webinar
Wed. 25 February 2026
1:00 pm EST (New York)
Live webinar
Wed. 25 February 2026
8:00 pm EST (New York)
Live webinar
Tue. 3 March 2026
11:00 am EST (New York)
Dr. Omar Lugo Cirujano Maxilofacial
Live webinar
Tue. 3 March 2026
8:00 pm EST (New York)
Dr. Vasiliki Maseli DDS, MS, EdM



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East










































To post a reply please login or register