Both intaglio and fitting surfaces can be contaminated by various agents to the detriment of the restoration’s prognosis. Some of the intrinsic contaminants to be considered are moisture from exhalation, ambient humidity in the oral cavity, blood and saliva.
Moisture is beneficial only when carefully controlled during the dentine penetration phase of substrate priming for adhesion. However, excessive moisture can interfere with the hybridisation process at the interface, compromising adhesion quality. Blood and saliva are ubiquitous in restorative dentistry and best controlled via the application of a dental dam.
Additionally, the particulate deposition of dentine and enamel during standard tooth preparation must also be considered. This smear layer remains on the dentinal surface and often occludes the dentinal tubules and must thus be removed for successful bonding to the hydroxyapatite and collagen fibrils of the surface.
Contamination of the prepared surface can also occur through extrinsic contaminants, such as provisional cement applied via a two-stage indirect delivery technique. Haemostatic agents such as ferric sulphate and aluminium chloride can deposit insoluble precipitates on the surface of the tooth that are only partially removable with a 33% phosphoric acid solution. Overall, the resulting risk of adhesive compromise or—in the worst case—failure, is high.
Intrinsic contaminants
Moisture is a critical component for maximising the adhesive bond strength of certain modern universal adhesives. The presence of moisture allows for increased penetration of bonding solutions into dentinal tubules and between collagen fibrils, ultimately bolstering the resilience of the hybrid layer.1
Van Meerbeek et al. reported that one-step contemporary universal adhesives require water as an ionisation medium for the self-etching reaction; however, these adhesives have been found to form interfaces semi-permeable to fluid.2 Given this and that HEMA has a high affinity for water, one-step adhesives containing HEMA increase the risk of hydrolytic degradation of the hybrid layer. Van Meerbeek et al. thus suggested that evaporating water from the interfacial surfaces may maximise bond strength.
Pereira et al. tested the effect of varying degrees of wetness of the dentinal substrate on bond strength, comparing various drying methods, including the use of short vs. long air blasts, wet vs. dry cotton pellets or a micro-brush, against leaving the surface overwet.3 In all groups, the wettest dentinal surface resulted in the lowest shear bond strength.
During the cementation of an indirect restoration, both salivary and blood contamination of the bonding surfaces have been shown to have a negative effect on bond strength.4 This is due to the deposition of salivary glycoprotein on the surface and macromolecules such as fibrinogen and blood platelets blocking access to the tubules for effective bonding. Blood contamination has consistently been found to be more deleterious to bond strength compared with saliva.4
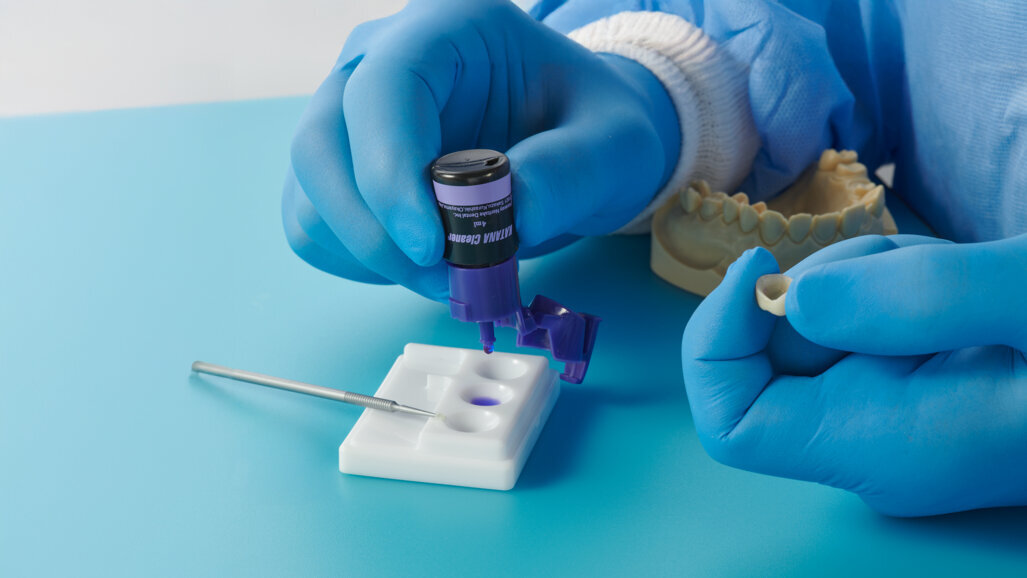
Salivary contamination of polycrystalline ceramic and metal alloy restorations upon try-in can be removed via steam cleaning and air abrasion set at 250 kPa for 15 seconds.5 Phosphoric acid is often mistakenly applied as a cleaning agent to the intaglio surface. In polycrystalline ceramics such as tetragonal zirconia polycrystal, this can significantly compromise bond strength, as phosphates will bond tightly to the free sites to which the 10-MDP monomer normally bonds as part of the APC protocol of zirconia bonding.6 Two studies of modern surface cleaners demonstrated successful debridement of the surface using KATANA Cleaner (Kuraray Noritake Dental), an MDP-based surfactant, for both blood- and saliva-contaminated substrates.7, 8
On dentine that had previously been etched and contaminated with blood and saliva, the subsequent application of 37.5% phosphoric acid has been shown to provide the highest recovery of shear bond strength.9 A study on the nanomechanical properties and nano-roughness of dentine etched with phosphoric acid and dentine treated with a self-etching adhesive, both contaminated with saliva, found that the KATANA Cleaner was capable of restoring these surfaces to the original values of untreated dentine.10 On smear layer-affected dentine, chlorhexidine has consistently shown better results than other agents, such as ethanol, EDTA and aloe vera, have in establishing the highest shear bond strength to dentine.
Synthetic workflow contaminants
Marfenko et al. demonstrated that salivary contamination of the intaglio surface of lithium disilicate restorations resulted in significantly lower bond strengths relative to contamination by dental stone from laboratory processes.11 The application of a silane coupling agent to the intaglio surface before contamination had a protective effect on the bond strength.
In this regard, it must be considered that lithium disilicate-based restorations are often requested pre-etched with hydrofluoric acid from the laboratory. Often, the case is returned to the clinician on the secondary or primary model. If already treated with hydrofluoric acid, the surface can be considered to have been re-contaminated by the stone or resin model, or simply by organic residues from handling. Given that it may thus contain elements of dental stone, blood and saliva, not to mention haemostatic agents such as aluminium chloride and ferric sulphate, the unprotected surface needs to be decontaminated after the try-in procedure. If silane coupling agents are applied prior to try-in, the question arises of whether the intaglio surface was truly contaminant-free after removal from the model.
Haemostatic agents used in clinical dentistry exhibit a pH of 1.1–3.0 and are as acidic as self-etching primers.12 Chaiyabutr and Kois found that, when contaminated with 25% aluminium chloride or 13% ferric sulphate, dentine demonstrated a significantly lower shear bond strength to self-adhesive resin cement and that this was significantly recovered using the etch-and-rinse approach.13 KATANA Cleaner was found to have a positive effect on the cleaning of dentine contaminated with aluminium chloride and ferric sulphate.
Because the insoluble precipitate that aluminium chloride leaves on the surface of dentine is only partially removed when treated with phosphoric acid, this achieves only a partial recovery of shear bond strength. The application of EDTA has been found to restore this bond strength to the level of normal dentine.14

CLEARFIL Universal Bond Quick.
Provisional cements are thought to have a deleterious effect on the shear bond strength of adhesively bonded indirect ceramic restorations. Ding et al. reported that use of resin-based and non-zinc oxide eugenol cements in the provisional phase decreased the bond strength and that the use of calcium hydroxide and polycarboxylate cements exhibited acceptable metrics.15 Debridement of the prepared surface with air abrasion resulted in recovery of decreased bond values. Equally useful was the application of immediate dentine sealing.16 This technique is characterised by air abrasion before adhesive bonding and the application of a resin coating, occluding both the dentinal tubules and isolating the oxygen inhibition layer and allowing the resin–dentine bond to mature and strengthen in the absence of stresses. This approach is effective in minimising postoperative hypersensitivity and bacterial ingress and in optimising the shear bond strength, particularly when indirect ceramic restorations are concerned.17 Hardan et al. found that the shear bond strength was highest when immediate dentine sealing was completed using a three-step etch-and-rinse adhesive protocol.16
The bonding of dentinal substrate contaminated with root canal sealers is a concern for the integrity of core build-ups after endodontic treatment. Tian et al. found the use of KATANA Cleaner to be generally superior to 70% ethanol in the removal of a sealer based on zinc oxide eugenol and to perform equally to 70% ethanol in the removal of an epoxy resin-based sealer.18
Clinical case demonstration

Fig. 1
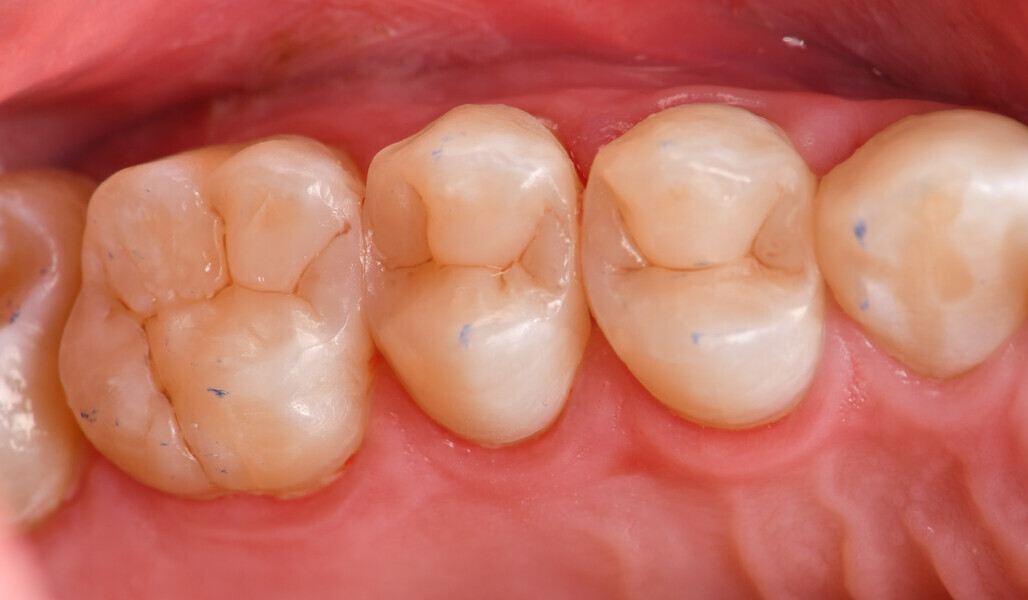
A 35-year-old female patient (ASA Class I) presented to the practice with multiple failing composite restorations in the maxillary left quadrant that were planned for replacement. Prior to the delivery of topical and local anaesthesia, it is a common procedure in the practice to ascertain shade specifics of planned restorations before dehydration affects the optical properties of the natural tooth. Smart monochromatic composites (Fig. 1) are a class of direct restorative materials that leverage the ability of their nano-filler composition and refractive index to mimic the structural colour of the surrounding enamel and dentin.19 This typically enables a clinician to have a simplified selection of shades on hand.
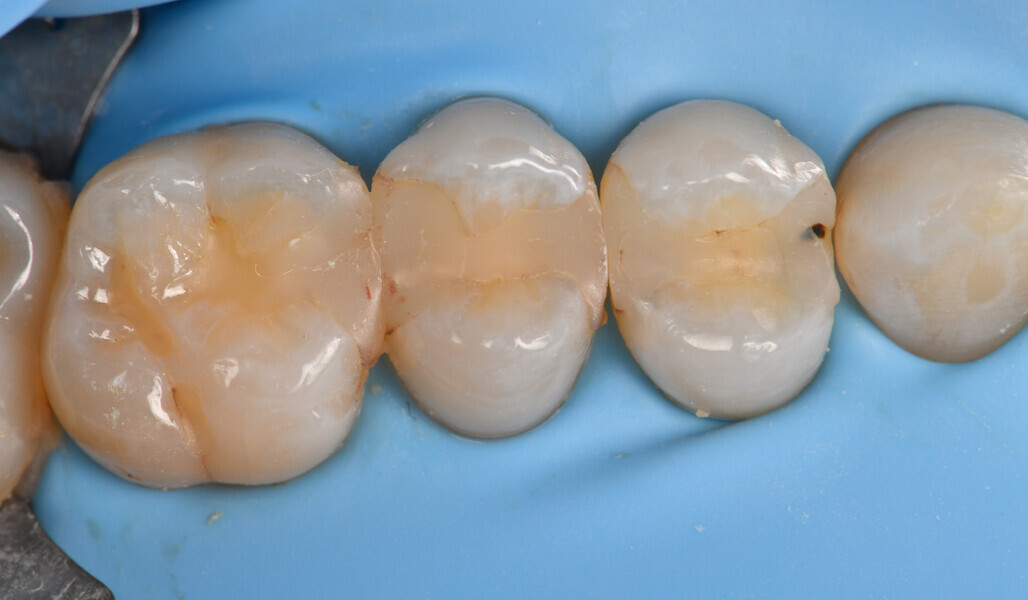
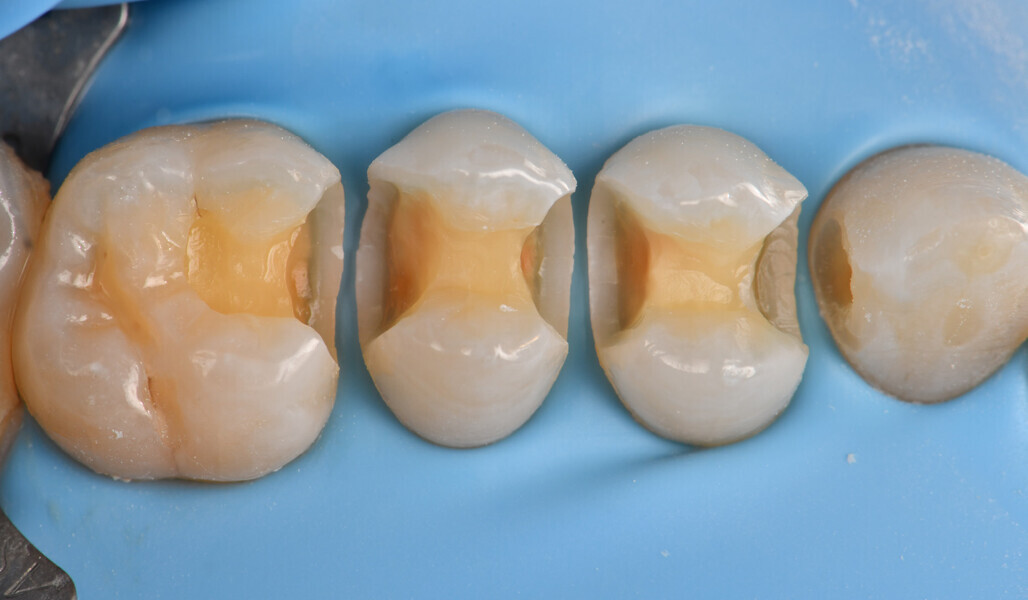
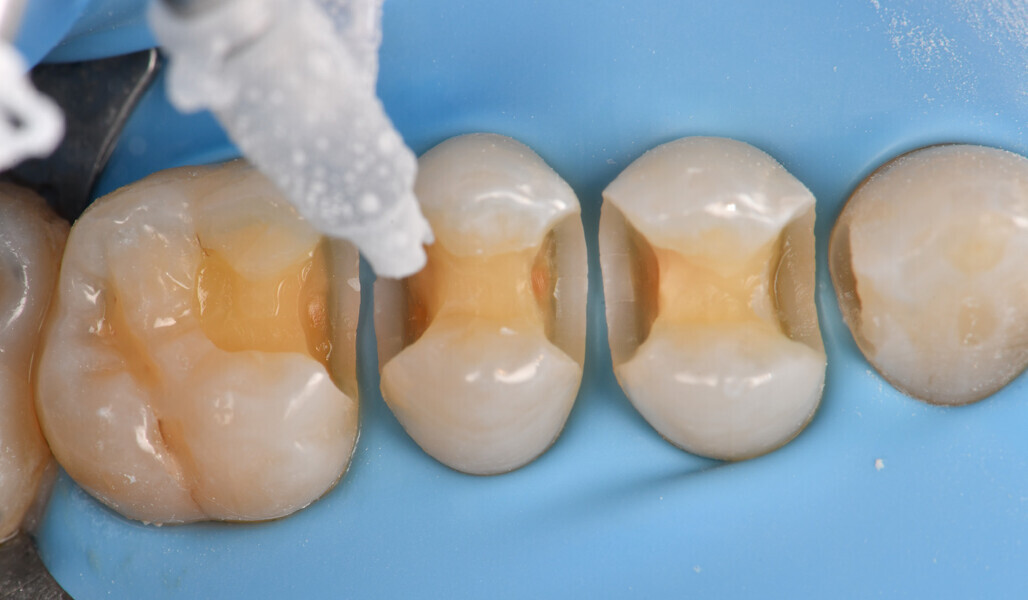
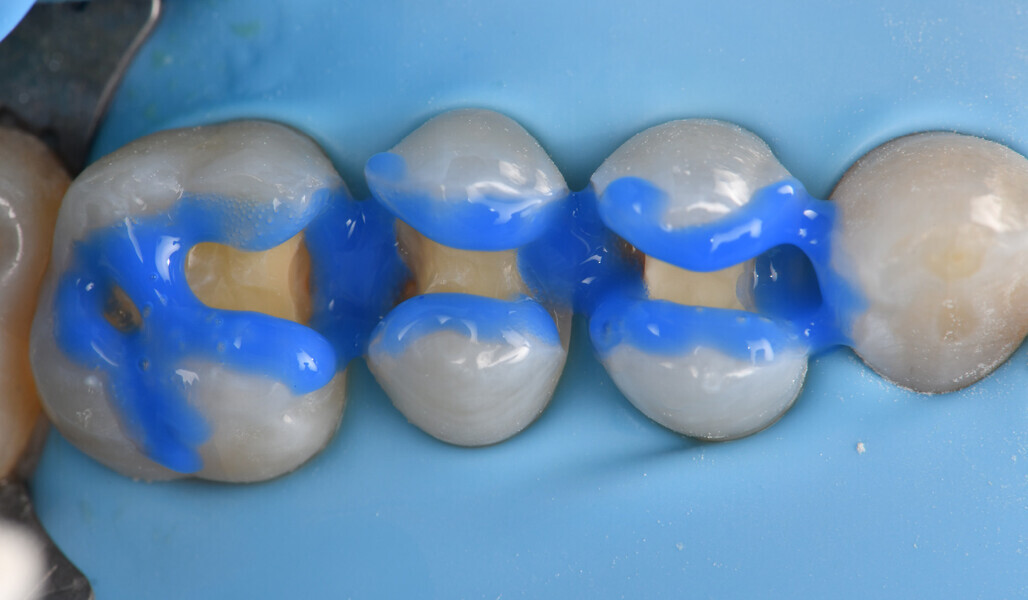
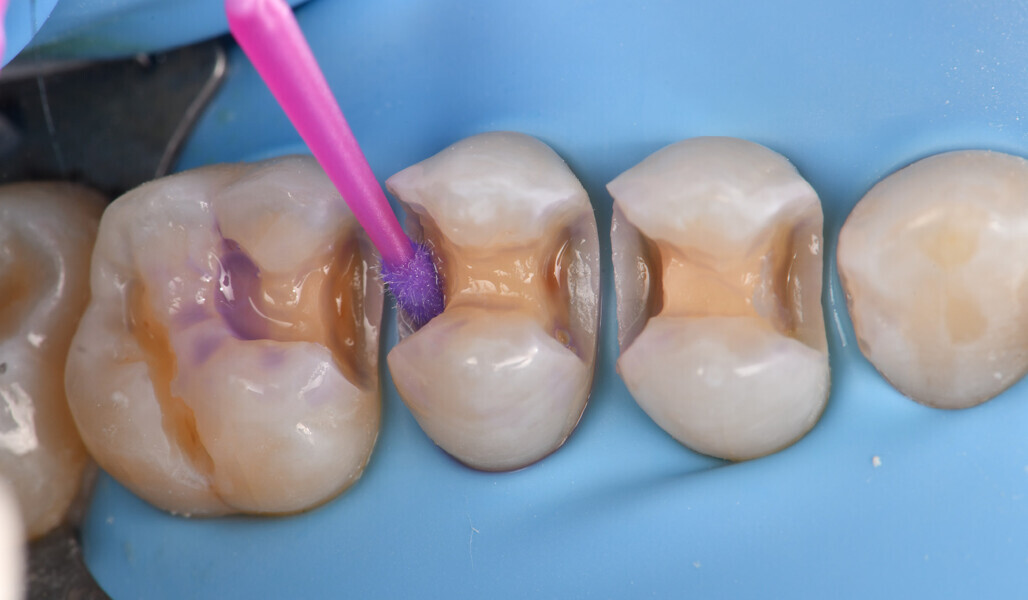
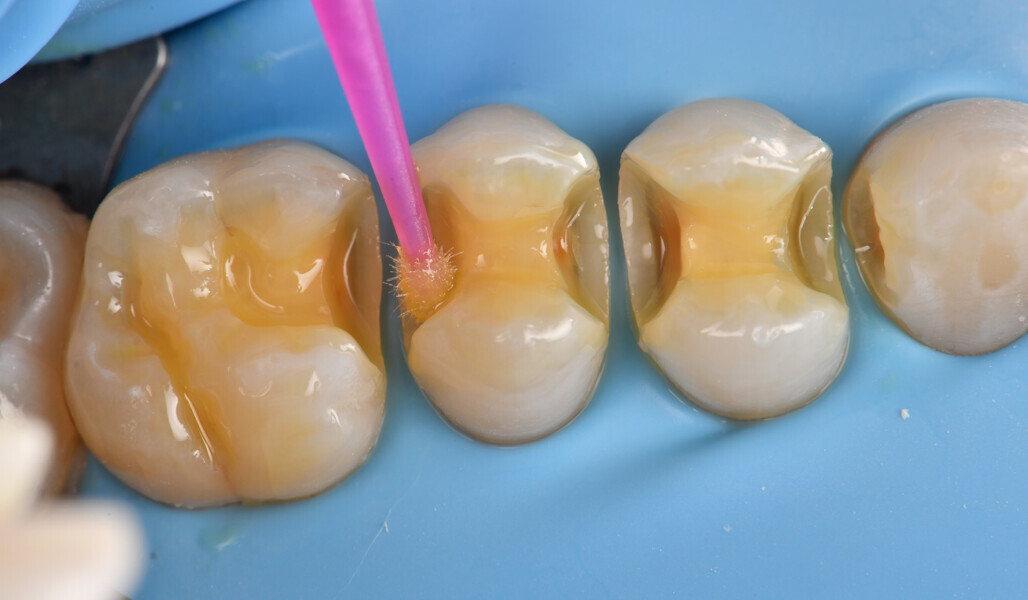
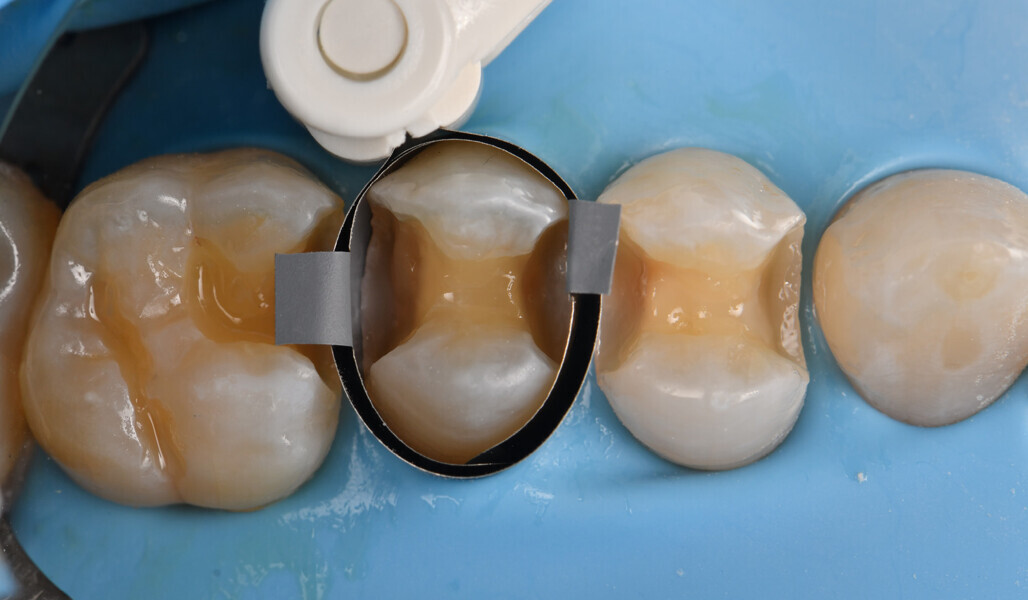
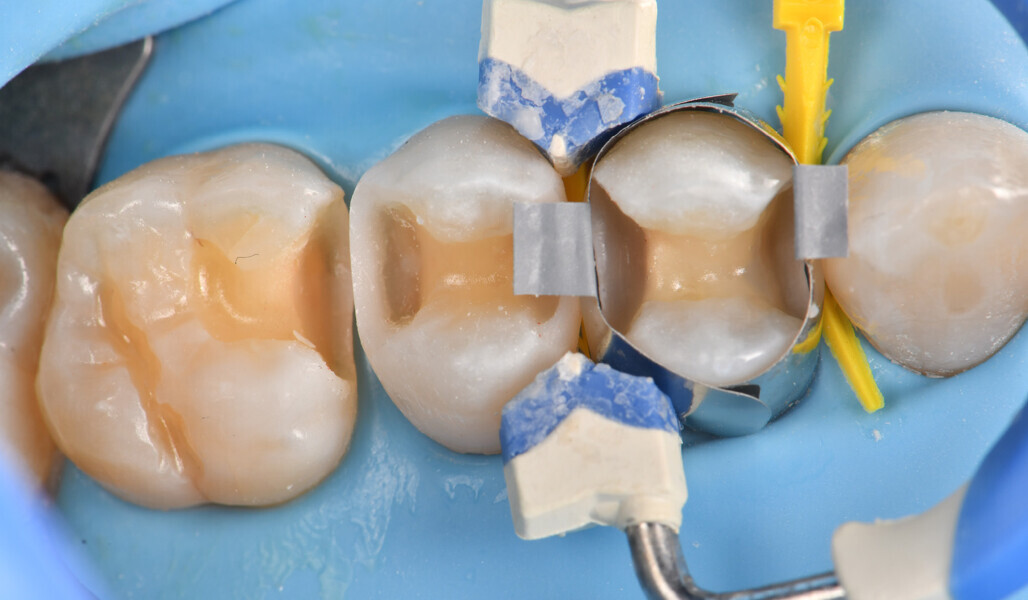
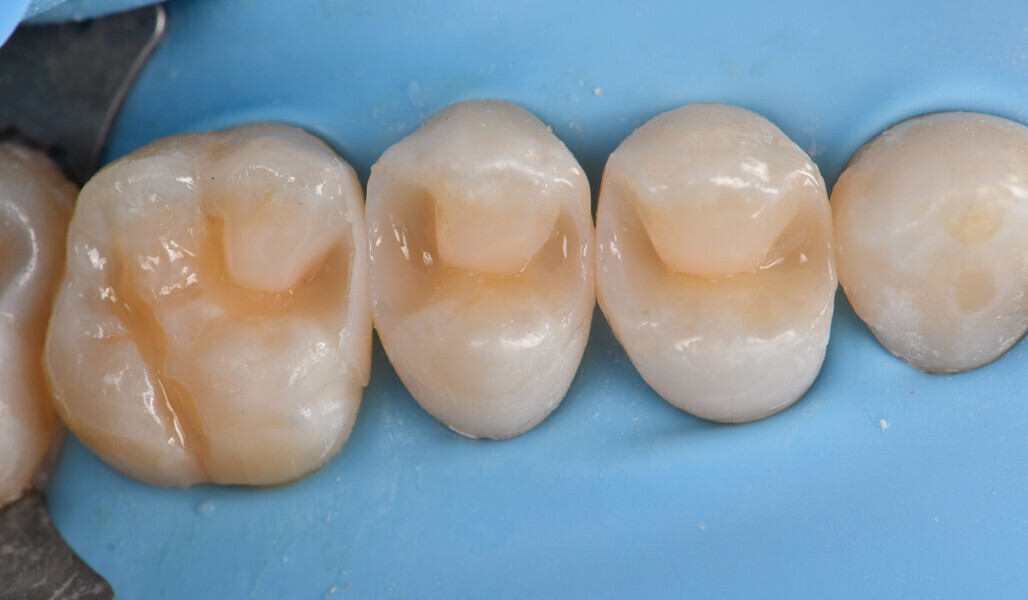
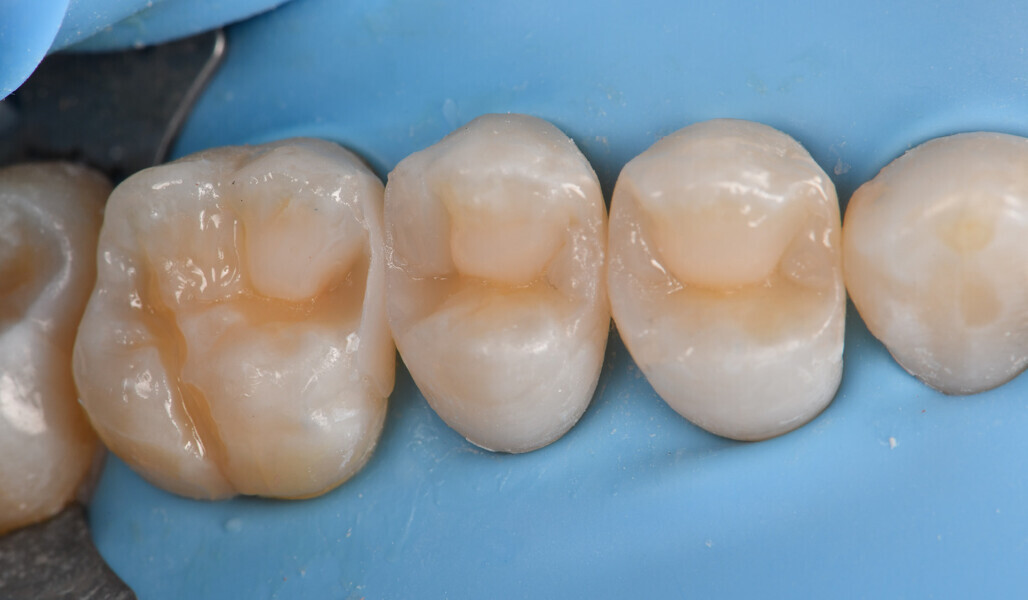
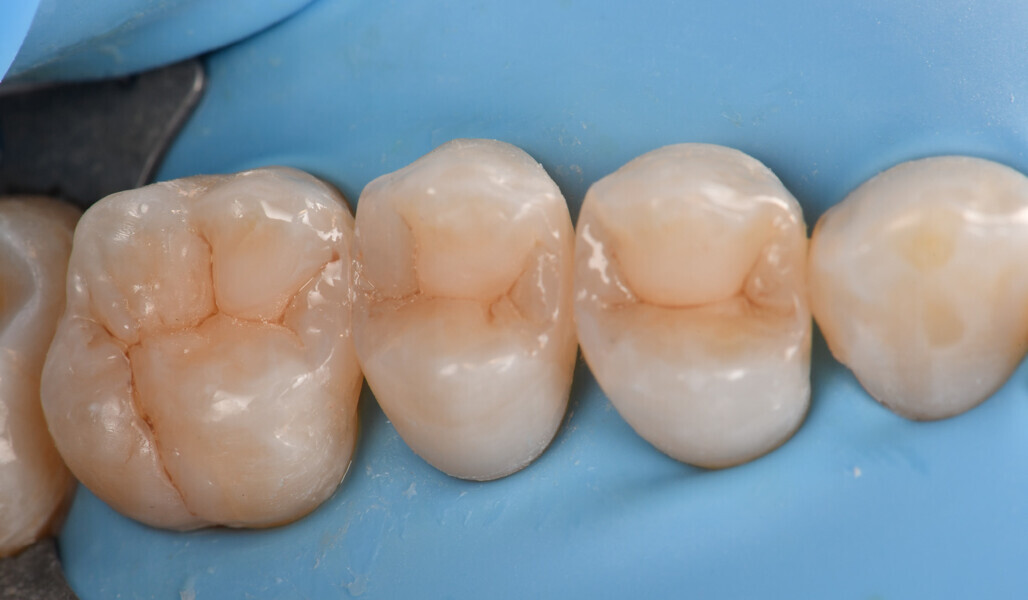
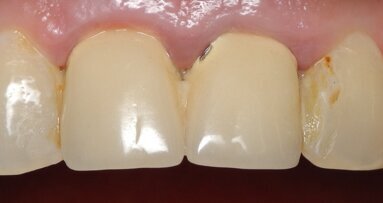
Two cartridges of 2% lidocaine with 1:100,000 adrenaline were delivered via buccal infiltration before absolute isolation was achieved using a non-latex dental dam (Isodam HD, Heavy; 4D Rubber; Fig. 2). The old restorations were excavated along with caries (Fig. 3), and the dentinal structure assessed for residual decay with a detector dye (CARIES DETECTOR, Kuraray Noritake Dental). The preparation cavosurface margins were gently bevelled before surface treatment with air abrasion (206.84 kPa; 29 μm aluminium oxide in a 17.5% ethanol carrier; AquaCare, Velopex International; Fig. 4). The enamel margins were etched with 33% phosphoric acid and rinsed (Fig. 5). The preparation surfaces were further decontaminated of any residual smear layer or powder residue using KATANA Cleaner (Fig. 6). A single-step self-etching universal adhesive was applied to the preparation according to the manufacturer’s instructions and air thinned before light polymerisation (Fig. 7).



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East





































To post a reply please login or register