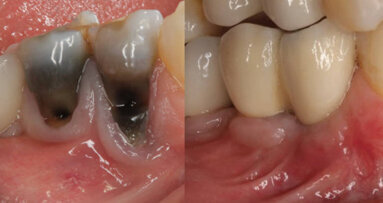
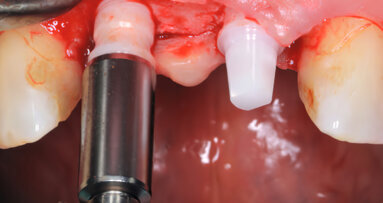
BERLIN, Germany: The CleanImplant Foundation, an established authority in evaluating the quality and cleanliness of implant surfaces, has awarded its Certified Production Quality seal of excellence to Komet Custom Made, a German supplier and original design manufacturer of ceramic implants.
According to Dr Dirk U. Duddeck, managing director and head of research at the CleanImplant Foundation, scanning electron microscope (SEM) analyses of more than 100 different commercially available implant systems found that more than half demonstrated significant impurities.
“These contaminants on new, sterile-packaged implants, which are entirely preventable on the manufacturer’s side, unfortunately have clinical consequences and harm both practitioners and patients,” Dr Duddeck stated. “It is our responsibility to inform dentists accordingly and provide a stage for quality manufacturers,” he continued.
Quality assured by rigorous testing
Based on the established CleanImplant consensus guideline on the cleanliness of dental implants, the Certified Production Quality seal is only awarded by the independent non-profit organisation after an extensive testing process conducted in an accredited testing laboratory. This certification is awarded to contract manufacturers such as Komet Custom Made that produce high-quality implants for various trade labels.
“The certification by the CleanImplant Foundation for ceramic implants confirms the process reliability of the quality assurance measures in place at the company, including validated final cleaning and subsequent packaging in the clean room, and represents a further milestone in ensuring the overall ceramic competence of Komet Custom Made,” noted Carsten Cieslik, general manager of Komet Custom Made.
As part of the family-run company Brasseler, Komet Custom Made offers a range of services, including implant design expertise based on many years of experience, the sophisticated packaging and manufacturing of ceramic implants, and the provision of other material and equipment, including abutments and implantology drills.
A beneficial mark for implant suppliers
Suppliers of implants produced by companies carrying the Certified Production Quality seal can benefit from this marker in multiple ways. The initiation of testing to obtain the coveted Trusted Quality Mark is facilitated by the CleanImplant Foundation. Conceptualised as an orientation tool for dental professionals, this mark may be awarded only after SEM analysis of five randomly selected samples of the same type and a final peer review of all results.
Tags:
COLOGNE, Germany: This year at the International Dental Show (IDS), 3Shape is celebrating 25 years in CAD/CAM. However, it was first in 2005 that 3Shape ...
Centuries ago, dentistry identified mineral deposits, such as calculus, as the main cause of dental disease. Further research then recognised bacterial ...
The 30th edition of the UAE International Dental Conference and Arab Dental Exhibition (AEEDC Dubai) is presenting its most comprehensive scientific ...
BERLIN, Germany: On 6 January 2021, the CeramTec Group, a world innovation leader for advanced ceramics, was awarded the Certified Production Quality seal ...
Periapical implant lesions may be a cause of early failure of implants. The purpose of this article is to describe the surgical treatment of the periapical ...
LONDON, UK: Introduced in 2006, the whiteSKY system from bredent has proved its efficiency in clinical practice and scientific studies. At the congress of ...
In an interview with Dental Tribune International, Prof. Dr Katja Nelson talks about her findings on patients with oral cancer after rehabilitation with ...
The figures speak for themselves. Last year’s AEEDC Dubai attracted over 70,000 participants and featured more than 5,000 dental brands. The world’s ...
KREUZLINGEN, Switzerland: For the third time this year, the CleanImplant Foundation has awarded its Trusted Quality Mark for outstanding surface ...
VIENNA, Austria: Residues on sterile packaged implants, in particular, organic particles from the production or packaging process, are strongly suspected of...
Live webinar
Wed. 4 March 2026
12:00 pm EST (New York)
Munther Sulieman LDS RCS (Eng) BDS (Lond) MSc PhD
Live webinar
Wed. 4 March 2026
1:00 pm EST (New York)
Live webinar
Wed. 4 March 2026
8:30 pm EST (New York)
Lancette VanGuilder BS, RDH, PHEDH, CEAS, FADHA
Live webinar
Fri. 6 March 2026
3:00 am EST (New York)
Live webinar
Mon. 9 March 2026
12:30 pm EST (New York)
Live webinar
Mon. 9 March 2026
3:00 pm EST (New York)
Live webinar
Tue. 10 March 2026
4:00 am EST (New York)
Assoc. Prof. Aaron Davis, Prof. Sarah Baker



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East


































To post a reply please login or register