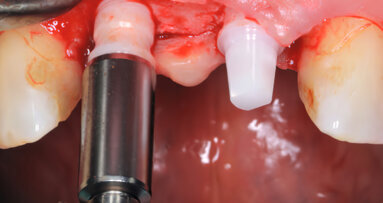
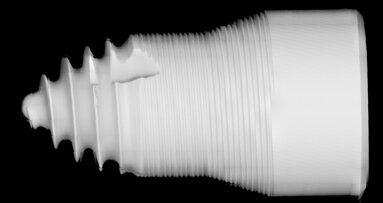
VIENNA, Austria: Residues on sterile packaged implants, in particular, organic particles from the production or packaging process, are strongly suspected of being responsible for incomplete osseointegration of dental implants or even loss of bone during the early healing period. Therefore, the CleanImplant Foundation established a new quality seal in order to provide increased safety for patients and practitioners. An implant from dental provider Bredent has been awarded the foundation’s “Trusted Quality” mark during the EAO congress.
Michael Norton, past President of the Academy of Osseointegration, summed up a problem in the implant market, “Dentists have to rely on the word of manufacturers and the FDA or CE marks to feel sure that the implants they are using are being manufactured to a standard one would expect of an implantable dental device. Sadly, this is often not the case.”
Four consecutive studies revealed alarming remnants and contaminants in the SEM-based quality assessment. Areal pollution and particles with iron, copper, chromium, nickel, tungsten, sulphur and large quantities of stainless-steel particles, as well as remnants of polytetrafluoroethylene and other significant organic contaminations gave cause for concern.
At the EAO Congress 2018 the CleanImplant Foundation awarded the Bredent group with the Trusted Quality Certificate. (Photograph: Timo Krause, OEMUS MEDIA)
Dr Dirk Duddeck hands over the CleanImplant Award to Peter Brehm, CEO of the Bredent group. (Photograph: Timo Krause, OEMUS MEDIA)
(Photograph: Timo Krause, OEMUS MEDIA)
(Photograph: Timo Krause, OEMUS MEDIA)
The Bredent group at EAO 2018 in Vienna. (Photograph: Timo Krause, OEMUS MEDIA)
(Photograph: Timo Krause, OEMUS MEDIA)
With the variety of implant systems offered on the market, it has become increasingly difficult for the dentist to choose a safe system for the practice. Based on a consensus paper signed by renowned scientists, the independent non-profit CleanImplant Foundation established a new global quality seal providing exactly this information. Implant companies with their systems already carrying the “Trusted Quality” mark, such as MIS V3, MegaGen AnyRidge, BTI UnicCa, NucleOSS T6 and NDI Replicate are presenting their tested products at the EAO 2018. In addition, other implant systems are currently in the process of examination. The most recent implant manufacturer that has been awarded is Bredent, based in Senden in Germany, for their implant system blueSKY at the EAO. The scientific advisory board proved compliance with the criteria required for issuing the quality mark. The official handover of the certificate took place at the Bredent booth.
From implant sample acquisition to environmental requirements necessary for unpacking and the subsequent analysis process, the CleanImplant Foundation guarantees unbiased research at a high scientific level.
Practitioners interested in a personalised certificate for their specific implant system, and implant manufacturers who want to apply for the new quality mark can find more information and a correspondent newsletter on the project's homepage at www.cleanimplant.com.
LONDON, UK: Introduced in 2006, the whiteSKY system from bredent has proved its efficiency in clinical practice and scientific studies. At the congress of ...
KREUZLINGEN, Switzerland: For the third time this year, the CleanImplant Foundation has awarded its Trusted Quality Mark for outstanding surface ...
BERLN, Germany: The CleanImplant Foundation has awarded the Kontact S implant system from French manufacturer Biotech Dental with the Trusted Quality Mark. ...
MONACO: Osstem Implant has announced its participation in the 32nd Annual Scientific Meeting of the European Association for Osseointegration (EAO 2025), ...
WASHINGTON, US: The CleanImplant Foundation recently introduced its non-profit initiative at the 11th annual meeting of the International Association of ...
LISBON, Portugal: Dentsply Sirona has demonstrated the latest developments in its continually evolving Astra Tech Implant System at the 2019 European ...
VIENNA, Austria: EAO President Dr Alberto Sicilia Felechosa took the stage at the opening ceremony of the 27th Annual Scientific Meeting of the EAO and ...
LOS ANGELES, U.S.: With the 33rd annual meeting of the Academy of Osseointegration (AO) taking place in Los Angeles, attendees are eagerly awaiting the ...
The CleanImplant Foundation seeks to promote global awareness of contaminated implant surfaces caused by poor quality control during production and ...
COMO, Italy: In November last year, 21 experts from 16 countries gathered in Como for a day of lectures, discussions and debate. The Ambassadors’ Summit, ...
Live webinar
Tue. 24 February 2026
1:00 pm EST (New York)
Prof. Dr. Markus B. Hürzeler
Live webinar
Tue. 24 February 2026
3:00 pm EST (New York)
Prof. Dr. Marcel A. Wainwright DDS, PhD
Live webinar
Wed. 25 February 2026
11:00 am EST (New York)
Prof. Dr. Daniel Edelhoff
Live webinar
Wed. 25 February 2026
1:00 pm EST (New York)
Live webinar
Wed. 25 February 2026
8:00 pm EST (New York)
Live webinar
Tue. 3 March 2026
11:00 am EST (New York)
Dr. Omar Lugo Cirujano Maxilofacial
Live webinar
Tue. 3 March 2026
8:00 pm EST (New York)
Dr. Vasiliki Maseli DDS, MS, EdM



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East












































To post a reply please login or register