This is a question more and more practitioners are hearing from their patients after the Implant Files, an investigation by an international consortium of 250 journalists, revealed a shocking lack of safety of dental implants and transparency in implanted medical devices in general. Patients are entirely right to be concerned, as current studies have shown significant impurities on numerous sterile-packaged dental implants. Dentists should not be lulled into a false sense of safety: neither the size and reputation of the manufacturer nor the country of manufacture can assure that implants are delivered as clean as promised or shown in advertising brochures.
Dr Michael Norton, past President of the Academy of Osseointegration, summed up the problem in the implant market: “Dentists have to rely on the word of manufacturers and the FDA [US Food and Drug Administration] or CE marks to feel sure that the implants they are using are being manufactured to a standard one would expect of an implantable dental device. Sadly, this is often not the case.”
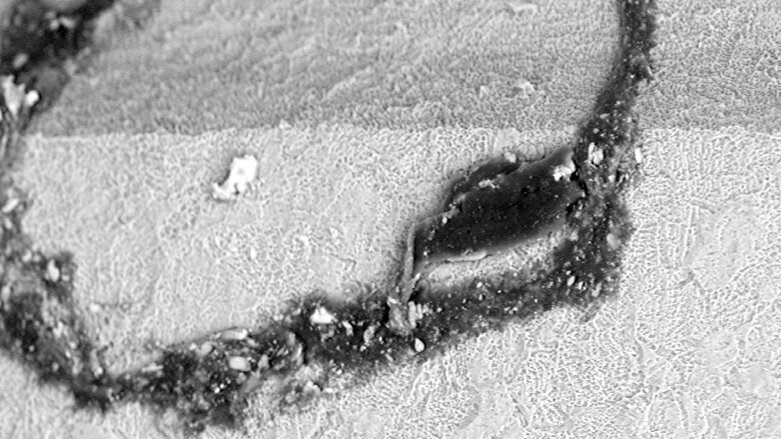
Impurities on sterile-packaged implants, in particular organic particles from the production or packaging process, are highly suspected of being responsible for incomplete osseointegration of dental implants or even loss of bone in the early healing period. And even worse, this has already given patients’ lawyers opportunity for negligence claims.
A recent study, initiated by the non-profit CleanImplant Foundation in close collaboration with the Charité—Universitätsmedizin Berlin in Germany, revealed a continually growing number of implants with severe contamination, compared with preceding analyses. Areal organic contaminants, plastic particles from handling and packaging, and particles of tungsten, tin bronze and PTFE, and even large quantities of stainless-steel particles on the sterile implant surface are cause for concern.
How can the clinician know which implants are not affected by impurities?
It has become increasingly difficult for the dentist to choose a safe system for his or her practice. The CleanImplant Foundation has set the goal of providing precisely this information worldwide. This independent organisation is supported and managed by a scientific advisory board, which is chaired by renowned scientists and practitioners. In 2017, the board set the criteria for a new global quality seal, the CleanImplant Trusted Quality Mark. Through a thorough protocol, implants are analysed in accredited testing laboratories according to DIN EN ISO/IEC 17025. This procedure guarantees objective results and reliable information for the new global quality seal.
Implant systems to date that meet the high standards of the Trusted Quality Mark are NobelActive (Nobel Biocare), V3 (MIS Implants Technologies), AnyRidge (MegaGen), UnicCa (BTI), SLA (Straumann), T6 (NucleOSS), blueSKY (bredent) and REPLICATE (NDI). Other implant systems are currently in the process of being analysed.
Practitioners interested in an appropriate answer to patients’ questions can join the CleanImplant Community and ask for a personalised certificate, which informs patients already in the practice waiting room. Implant manufacturers who want to apply for the quality mark can find more information on the project’s website, www.cleanimplant.com.
At IDS in Cologne in Germany, the CleanImplant Foundation will be demonstrating full transparency and showing whether implants are as clean as promised by the respective manufacturers with a scanning electron microscope (SEM) installation on-site. On Wednesday, 13 March, at 5 p.m., the organisation will be holding a lecture at the IDS Speakers’ Corner explaining why “sterile” does not necessarily mean “clean” and why there is a need for a global quality mark in implant dentistry.
Visitors to IDS can learn more about this in Hall 11.1 at Booth B020–C021.
GOTHENBURG, Sweden: Single-tooth implants have demonstrated high success rates over observation periods of five to ten years. However, long-term monitoring...
COLOGNE, Germany: Dentium is a leading manufacturer of dental implant systems and solutions, used by clinicians in more than 70 countries worldwide. As an ...
CHICAGO, U.S.: The American Association of Endodontists (AAE) is devoting an entire month to celebrating saving natural teeth and recognizing the ...
Conducted in collaboration with the Medical University of Graz in Austria, a new independent study followed patients treated with the two-piece Patent ...
Implant dentist and key opinion leader Dr Marco Tudts is one of the leading minds behind the development of edelweiss dentistry’s Ceramir CAD/CAM block. ...
SEOUL, South Korea: Osstem Implant’s OneGuide, a unique surgical guide for guided surgery, does not require the production of separate guides for ...
COLOGNE, Germany: Dental implant surfaces are continuously being improved to achieve better and faster integration with the bone. However, a study being ...
BIETIGHEIM-BISSINGEN, Germany: Owing to the large number of patients seen in dental practices, there is always a danger of coming into contact with an ...
BEIJING, China: Autonomous dental implant robotic systems (ADIRSs) represent an exciting and innovative advancement in implant dentistry, offering ...
EDMONTON, Canada: A team of researchers at the University of Alberta has secured funding to develop a 3D ultrasound device that would allow dentists to ...
Dr. Vasiliki Maseli DDS, MS, EdM
Live webinar
Wed. 4 March 2026
12:00 pm EST (New York)
Munther Sulieman LDS RCS (Eng) BDS (Lond) MSc PhD
Live webinar
Wed. 4 March 2026
1:00 pm EST (New York)
Live webinar
Wed. 4 March 2026
8:30 pm EST (New York)
Lancette VanGuilder BS, RDH, PHEDH, CEAS, FADHA
Live webinar
Fri. 6 March 2026
3:00 am EST (New York)
Live webinar
Tue. 10 March 2026
4:00 am EST (New York)
Assoc. Prof. Aaron Davis, Prof. Sarah Baker
Live webinar
Tue. 10 March 2026
8:00 pm EST (New York)
Dr. Vasiliki Maseli DDS, MS, EdM



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East


































Good presentation. Must differentiate between sterile and clean!!!