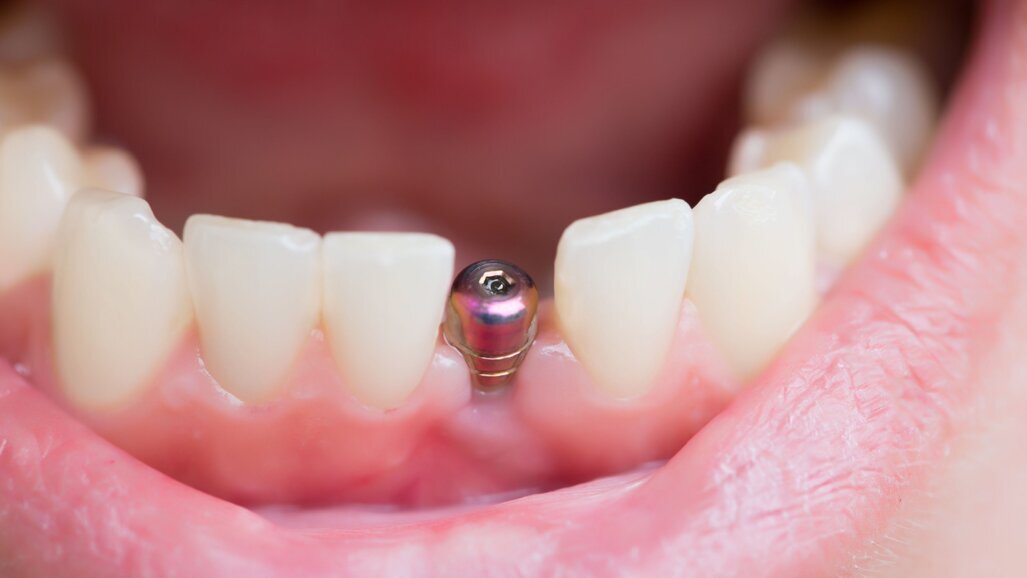
CHICAGO, US: Although manufacturers indicate that healing abutments (HAs) are single-use components, HAs are sometimes reused in clinical practice. Academic institutions being at the forefront of evidence-based dentistry, a study has looked into the prevalence of reuse of HAs across American periodontics residency programmes and their decontamination protocols, as concerns have arisen about their reuse owing to potential cross-contamination and the risk of initiating inflammatory responses in patients. The researchers found that, although only a small number of programmes reuse HAs, the lack of standardisation of decontamination practices could put patients at risk of exposure to biomaterials.
The researchers utilised a survey distributed to programme directors of 57 accredited periodontics residency programmes in the US. The survey included seven questions focused on the reuse of HAs, the duration for which they are left in situ and the decontamination techniques employed.
Among the 14 programmes that completed the survey, three reported reusing HAs. The responses revealed an almost even split between one-stage and two-stage implant placement protocols. The duration that HAs remained in situ varied widely, from four weeks to six months, depending on the case specifics and the protocol followed.
Decontamination techniques included manual cleaning, ultrasonic cleaning, rinsing and heat sterilisation in autoclaves. However, the lack of standardisation in the methods used raises concerns, given the results of multiple studies cited in the paper that demonstrated incomplete removal of biomaterials from HA surfaces after standard cleaning procedures and autoclave sterilisation in clinical practice. This residual contamination poses a risk of cross-patient contamination and could lead to adverse biological responses, such as inflammation, which may compromise the success of the implant.
Furthermore, the US Centers for Disease Control and Prevention and Food and Drug Administration imply that HAs should be treated as single-use devices and thus should not be reused. Despite this, economic pressures in educational settings may influence the decision to reuse these components, potentially compromising patient safety. Additionally, the ethical and legal implications of reusing single-use devices, especially without patient consent, are significant and could expose institutions to litigation.
The findings of this study suggest that the reuse of HAs is not widespread in periodontics residency programmes. However, the authors emphasised that further research is needed to assess the true extent of HA reuse in both educational and private practice settings. Moreover, there is an urgent need to establish standardised, effective decontamination protocols to ensure that reused HAs do not pose a risk to patients.
The study, titled “Are healing abutments being reused in periodontics residency programs in the United States? A survey-based study”, was published online on 4 July 2024 in the Journal of Dental Education, ahead of inclusion in an issue.
Topics:
Tags:
TAIYUAN, China: Over the last couple of weeks, details have begun emerging of an investigation into a disturbing and highly profitable trade in dead body ...
The initial phase of the biological response to a placed implant is primarily determined by the implant’s surface characteristics. The properties of any ...
LOS ANGELES, US: With reported cases as high as 19%, the battle against peri-implantitis has left clinicians and researchers constantly searching for ways ...
GENEVA, Switzerland: The non-profit CleanImplant Foundation will be present at the 29th Annual Scientific Meeting of the European Association for ...
WARDHA, India: Health authorities in India face several obstacles in the efficient delivery of dental care, including a dentist-to-population ratio that is ...
LONDON, UK: The SKY fast & fixed implant of bredent improves bone quality and quantity and reduces the risk of complications. The majority of patients ...
Live webinar
Wed. 25 February 2026
8:00 pm EST (New York)
Live webinar
Tue. 3 March 2026
11:00 am EST (New York)
Dr. Omar Lugo Cirujano Maxilofacial
Live webinar
Tue. 3 March 2026
8:00 pm EST (New York)
Dr. Vasiliki Maseli DDS, MS, EdM
Live webinar
Wed. 4 March 2026
12:00 pm EST (New York)
Munther Sulieman LDS RCS (Eng) BDS (Lond) MSc PhD
Live webinar
Wed. 4 March 2026
1:00 pm EST (New York)
Live webinar
Fri. 6 March 2026
3:00 am EST (New York)
Live webinar
Tue. 10 March 2026
4:00 am EST (New York)
Assoc. Prof. Aaron Davis, Prof. Sarah Baker



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East































To post a reply please login or register