COLOGNE, Germany: Nobel Biocare has invited dental professionals to join the Mucointegration era with the launch of the Xeal and TiUltra surfaces at IDS 2019. Applied to not only implants but also abutments, these new surfaces have been created to optimise tissue integration at every level for the purpose of improving implant treatment outcomes.
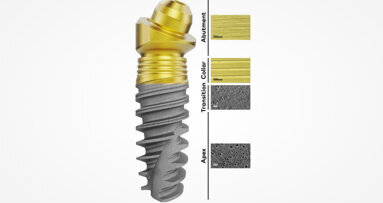
With decades of expertise in applied anodisation technology, Nobel Biocare has recognised that, for the optimal integration of dental implant restorations, different tissues demand different surfaces. While osseointegration is fundamental for the long-term function of implant restorations, soft-tissue integration is often overlooked. Dense soft-tissue contact with the abutment, however, can act as a barrier to protect the underlying bone.
To maintain long-term tissue health and stability, the surface chemistry and topography of the new Xeal abutment were specially designed to facilitate soft-tissue attachment to the abutment. Backed by data from a clinical study with two-year follow-up, the Xeal surface has demonstrated a significant increase in soft-tissue height compared with machined abutment surfaces. This Mucointegration surface is available for the On1 Base and Multi-unit Abutment from Nobel Biocare, and dental professionals can see and experience Xeal in Cologne.
Developed with the ultimate goal of early osseointegration and long-term bone stability in mind, the ultra-hydrophilic, multi-zone implant surface TiUltra takes anodisation technology a step further. Going beyond just roughness, the surface chemistry was developed with the aim of positively influencing its interaction with cells and ultimately osseointegration. TiUltra’s topography has also been reimagined to change gradually from a minimally rough and non-porous collar to a moderately rough and porous implant apex, thus respecting the natural transition from hard, dense cortical bone to cancellous bone.
The TiUltra surface will be available for Nobel Biocare’s NobelActive and NobelParallel Conical Connection implants. Combined with the Xeal abutment surface, this will give dental professionals a new complete solution for soft-tissue health, bone protection and fast osseointegration.
From left to right: Prof. Stefan Holst, Vice President Global Research, Products and Marketing at Nobel Biocare; Dr Axel Kirsch, Principal Managing Partner at LOGON; Dr Pascal Kunz, Vice President Digital Solutions at Nobel Biocare; and Hans Geiselhöringer, President and CEO of Nobel Biocare. (Photograph: Brendan Day, DTI)
The Nobel Biocare booth. (Photograph: Tom Carvalho, DTI)
(Photograph: Tom Carvalho, DTI)
Nobel Biocare President and CEO Hans Geiselhöringer. (Photograph: Tom Carvalho, DTI)
(Photograph: Tom Carvalho, DTI)
The Nobel Biocare booth showing the new Nobel Biocare logo. (Photograph: Tom Carvalho, DTI)
To ensure that every implant and abutment is delivered in pristine condition, the surface chemistry and hydrophilicity of both Xeal and TiUltra are preserved with a protective layer that dissolves upon contact with fluids.
“Having mastered osseointegration for decades, Nobel Biocare is taking tissue integration to a new level. With the launch of Xeal and TiUltra at IDS Cologne, we are not only advancing our leadership position in surface technology but also introducing a new era for implant dentistry—that of Mucointegration,” commented Hans Geiselhöringer, President of Nobel Biocare, at the launch. “Our focus has always been on the patient. These new solutions are supported by clinical evidence, including a two-year clinical trial.”
Dental professionals in CE markets will be the first to gain access to the new surfaces, in April, followed by other regions around the globe.
An insight into the scientific evidence already available on the new surfaces will be published in a dedicated supplement of Clinical Implant Dentistry and Related Research, and seven studies are expected to be released online in March.
IDS attendees who wish to find out more about the features of Xeal and TiUltra are invited to visit Nobel Biocare's booth (#H020–J029) in Hall 10.1. The company also provides information online at nobelbiocare.com/surface.
Tags:
YORBA LINDA, Calif., U.S.: Nobel Biocare is inviting dental professionals to join what it calls the Mucointegration era with the launch of its Xeal and ...
An award-winning clinician, researcher and director at the Brånemark Centre in Perth in Australia, Dr Graham Carmichael is a consultant prosthodontist and ...
HALIFAX, Nova Scotia, Canada: A new clinic for Dalhousie University’s Faculty of Dentistry is set to be officially opened by the end of this month. ...
ZURICH, Switzerland: Peri-implantitis is one of the greatest unsolved problems in dental implantology, and its prevalence is expected to increase over time ...
MILAN, Italy: Navigated photogrammetry opens the door to a new dimension of prosthetic design for full-arch implant-borne restorations. On show at Nobel ...
LOS ANGELES, U.S.: SprintRay, a 3D printer manufacturer that focuses on digital dentistry, announced last week that it has commenced a partnership with the ...
ZURICH, Switzerland: Nobel Biocare and DOCERAM Medical Ceramics are combining their strengths in order to offer high-quality restorative solutions which ...
PARIS, France: At the congress of the European Association for Osseointegration (EAO), currently being held in Paris, dental implants manufacturer Nobel ...
ZURICH, Switzerland: Dental implantology leader Nobel Biocare has expanded its portfolio of regenerative products with the launch of creos syntoprotect, a ...
ZURICH, Switzerland: At its 2019 Global Symposium in Madrid, Nobel Biocare will present a new implant system designed to challenge conventional methods of ...
Dr. Annette Felderhoff-Fischer
Dr. Rafael Saviolo Moreira
Dr. Ian Lane Dip SED, BDS (Hons) (Ncl), MFGDP, MSc, KOIS Graduate



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East















































To post a reply please login or register